1. For any solution the partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction. Name the law stated above.
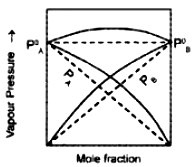
2. Study the graph. What phenomenon it denotes? Based on your observation predict the reason for the greater volatility of a mixture of carbon disulphide and acetone?