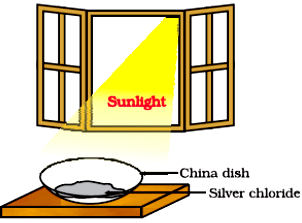
In the above image, 2 g silver chloride is taken in a china dish and is kept in sunlight, after sometime we observe that the colour of the silver chloride changes.
1. What is initial colour of silver chloride?
(i) White
(ii) Grey
(iii) Black
(iv) Yellow
2. What is final colour of silver chloride?
(i) White
(ii) Grey
(iii) Black
(iv) Yellow
3. What is the type of the chemical reaction occuring in the above experiment?
(i) Combination
(ii) Double displacement
(iii) Decomposition
(iv) None of the above
4. State true or false : Silver bromide also behaves in the same way like Silver chloride in presence of sunlight.
(i) TRUE
(ii) FALSE
5. Which type of reactions are used in black and white photography ?
(i) Combination
(ii) Displacement
(iii) Redox
(iv) Thermal decomposition