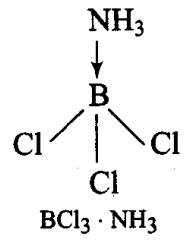
In BCl3, the central B atom has six electrons in the valence shell. It is, therefore, an electron deficient molecule and needs two more electrons to ‘ complete its octet. In other words, BCl3 acts as a Lewis acid. NH3, on the other hand, has a lone pair of electrons which it can donate easily. Therefore, NH3 acts as a Lewis base. The Lewis acid (BCl3) and the Lewis base (NH3) combine together to form an adduct as shown below:
In AlCl3, Al has six electrons in the valence shell. Therefore, it is an electron deficient molecule and needs two more electrons to complete its octet. Chlorine, on the other hand, has three lone pairs of electrons. Therefore, to complete its octet, the central Al atom of one molecule accepts a lone pair of electrons from Cl atom of the other molecule forming a dimeric structure as shown below.
