Five mole of a gas put through a series of change as shown below graphically in a cyclic process. The processes `A rarr B, B rarr C` and `C rarr A`, respectively, are
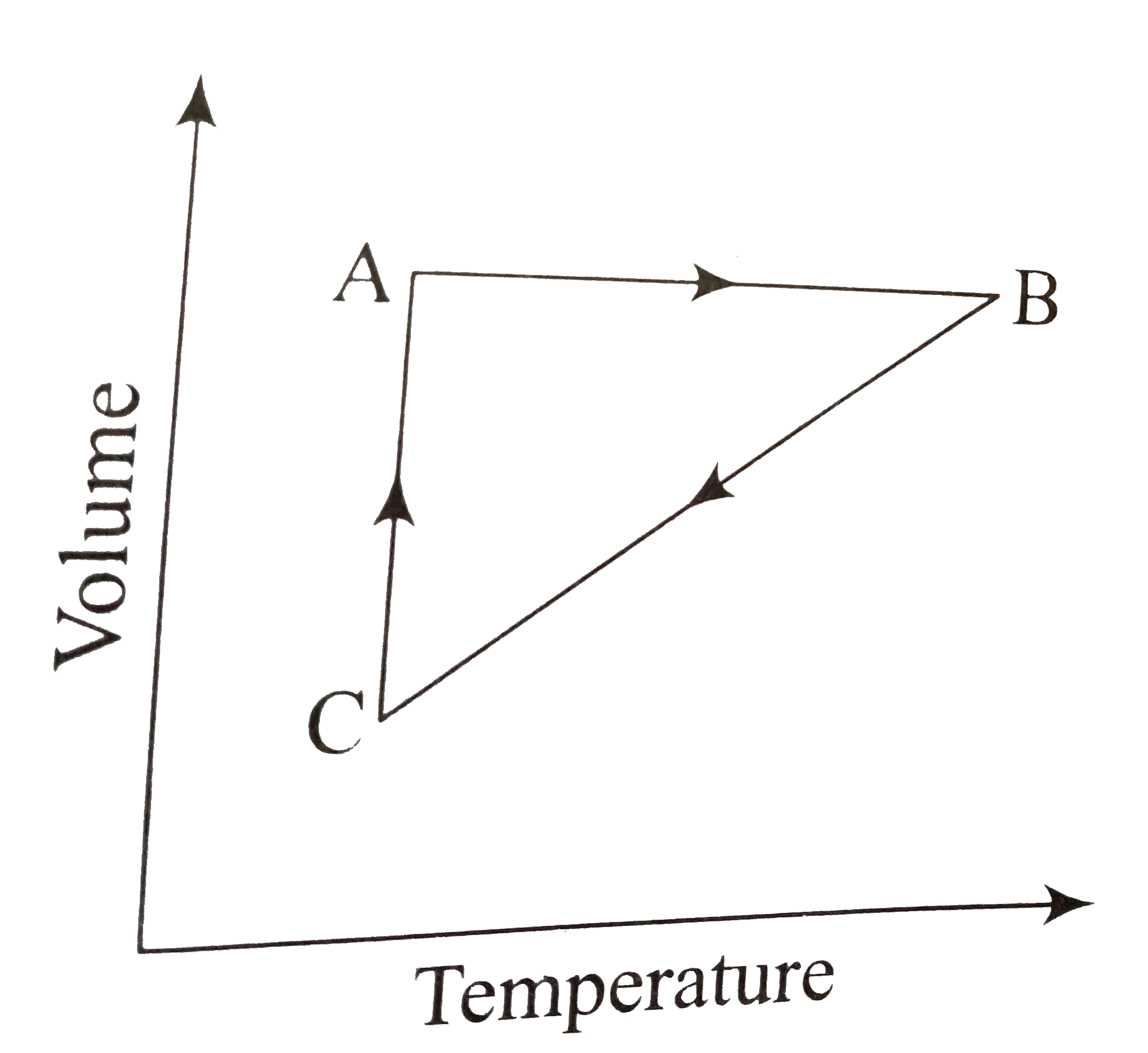
A. isochoric, isobaric, isothermal
B. isobaric, isochoric,isothermal
C. isothermal,isobaric,isochoric
D. isobaric, isothermal, isobaric