(a) Aluminium metal is rendered passive by nitric acid `(HNO_(3))` due to the formation of an oxide layer `(Al_(2) O_(3))` on its surface. Hence, `Al` vessel is not atteacked by `conc HNO_(3)` and `concHNO_(3)` can be easily stored in aluminium vessel.
(b) Both `BCl_(3)` and `AlCl_(3)` are electron-deficient compounds as both `B` and `Al` have only six electrons around them in `BCl_(3)` and `AlCl_(3)`, respectively and hence their octet is incomplete. To complete their octet, `p pi- p pi` back bonding is not possible in `AlCl_(3)` due to increase in size of `Al`. Hence `Al` completes its octet by forming dimer in which `Cl` atom of one `AlCl_(3)`, donates an electron pair in the vacant `3p` orbital of `Al` by forming a coordinate bond, whereas in `BCl_(3), p pi- p pi` back occurs and `BCl_(3)` exists as a monomer and not as a dimer.
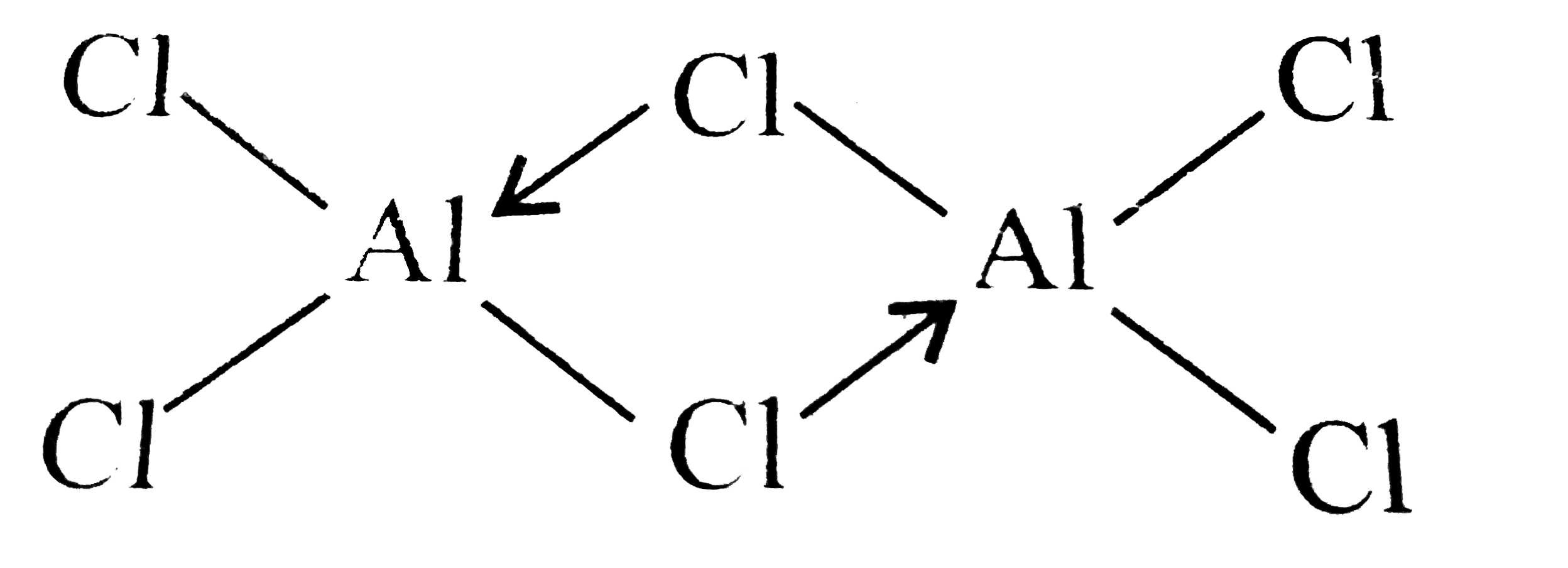
( c) Aluminium gas great affinity for oxygen. Aluminium thus removes oxygen from the oxides of less electropositive metals and as a good reducing agent.
`Cr_(2) O_(3) + 2Al rarr Al_(2) O_(3) + 2 Cr`
(d) Aluminium cannot be prepared by the electrolysis of aqueous solution of its salt, as discharge potential of aluminium is higher than the discharge potential of hydrogen. Thus, the aqueous solution containing `Al^(3+)` ions and `H^(oplus)` ions, when electrolysed, the `H^(oplus)` ions rather than `Al^(3+)` ions are discharge at cathode and hydrogen is liberated.
At cathode : `2H^(oplus) + 2e^(Ө) rarr H_(2) uarr`
( e) Due to `p pi - p pi` back bonding in `BX_(3)`, the molecule has double bond character, which results in shortening of `B-X` bond distance in `BX_(3)`.

(f) Despite the fact that ionsation potential of boron `(8.30 eV)` is less than gold `(9.22 eV)`, boron is a non-metal. This is due to difference is structure in the solid state. In general, metals have large number of atoms as neighbours as compared to non-metal. Gold has `12` atoms as neighbours, whereas boron has `6` or less atoms as neighbours in the solid state.