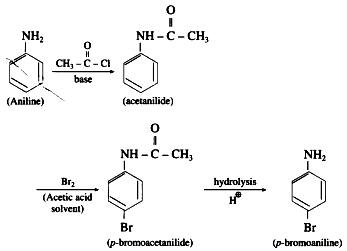
NH2 – group in aniline is highly ring activating and o – /p – directing due to involvement of the lone pair of electrons on ‘N’ in resonance with the ring. As a result, on reaction with Br it gives 2, 4, 6-tribromoaniline. To get a monobromo product, it is necessary to decrease the ring activating effect of – NH2 group. This is done by acetylation of aniline. The lone pair of ‘N’ in acetanilide is also involved in resonance in the acetyl group. To that extent, ring activation decreases.

Hence, acetanilide on bromination gives a monobromo product p-bromoacetanilide. After monobromination the original – NH group is regenerated. The protection of – NH group in the form of acetyl group is removed by acid catalyzed hydrolysis to get p-bromoaniline, as shown in the following scheme.