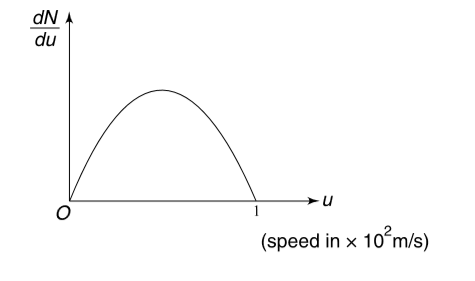
The speed distribution of molecules in a sample of a gas is shown in the figure. The graph between `(dN)/(du)` and u is a parabola and total number of molecules in the sample is `N_(0)`.
(a) Calculate the rms speed of the molecules.
(b) Calculate the total translational kinetic energy of molecules if mass of the sample is 10 g.