Do you ever thought that what if we start dividing matter till its last pace? This chapter deals with the solution of such confusions. In this article Structure of the atom Class 9 notes we will read about discovery of atoms, subatomic particles, discovery of electrons – Cathode ray experiment by JJ Thomson, protons – anode rays, Neutrons by James Chadwick, Atomic models of JJ Thomson, Rutherford, Neil Bohr, Atomic number, Mass Number, Distribution of electrons in different Shells, Isotopes and Isobars.
Introduction:
- John Dalton considered atom as the indivisible particle i.e. further it can’t be divided.
- After discovery of ‘subatomic particles,’ Dalton’s theory was discarded.
- Sub-atomic particles include Electrons, Protons and Neutrons.
- Further read this article on Structure of the atom class 9 notes as a revision material.
Dalton’s Atomic Theory:
- All matter was composed of atoms.
- Atoms are indivisible and indestructible
- All atoms of an element were identical, whereas atoms of different elements had differing size and mass.
- All compounds were composed of combinations of these atoms in defined ratios.
Limitations of Dalton’s Atomic Theory:
- Atom consists of particles like electrons, protons, neutrons etc. thus atom is divisible.
- Isotopes discovery revealed that atoms of the same element possess different weights.
Notes of Structure of the class 9 notes includes all the important topic based on CBSE curriculum.
Subatomic Particles:
| Synonyms |
Electrons |
Protons |
Neutrons |
|
Discovered by |
J.J Thomson |
E. Goldstein |
J. Chadwick |
|
Charge |
-1.6 x 10-19 C |
+ 1.6 x 10-19 C |
0 |
|
Mass |
9.1 x 10-31 Kg |
1.673 x 10-24 gm |
1.673 x 10-24 gm |
Structure of the atom:
Structure of the atom for class 9 CBSE notes is here with all the atomic models. Proposed models are given below:
Thomson’s Model of an atom:
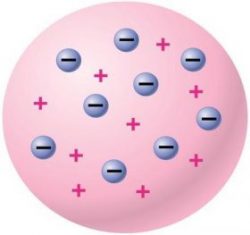
- By Cathode ray experiment Thomson explained the presence of electrons in atom.
- This model of atom is also known as plum pudding model.
- According to him, atom is a positively charged sphere and the electrons are embedded in it.
- The magnitude of positive and negative charge is same inside atom, so atom is neutral.
Limitations of Thomson’s Model of an atom:
- Electrically neutral behavior of atom was not explained well using this model.
- The further experiments deviated from the facts that were considered by J.J Thomson.
- Read and revise notes for Structure of the atom class 9.
Rutherford’s α - particle scattering experiment:
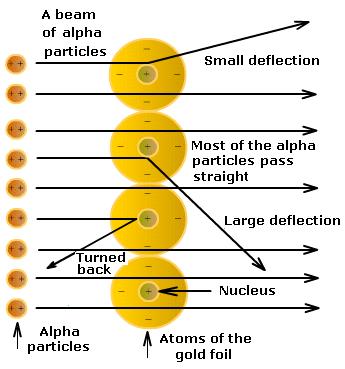
- In his experiment, he bombarded fast moving α-particle on thin gold foil and noted the observations.
- He selected a gold foil because thin layer was required. This gold foil was about 1000 atoms thick.
- α - particles are doubly-charged helium ions (He2+). They are highly energetic as they have 4 u mass.
Observations:
- Most of the α-particles passed straight through the gold foil.
- Some of the α-particles were deflected by small angles.
- One out of 12000 α particles returned back to the same path.
Conclusion of experiment:
- Most of the space inside an atom is empty.
- Positive charge occupies a little space in an atom.
- Inside an atom positive charge and mass of an atom is concentrated in a small volume (called nucleus).
- Radius of nucleus is 105 times less than the radius of the atom.
- Thus revise the terms in structure of the atom for class 9 regularly.
Rutherford’s model of an atom:
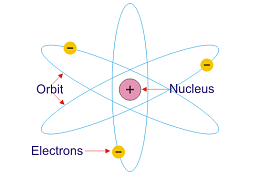
- There is a positively charged centre in an atom where all the mass of atom lies is called nucleus.
- The electrons of the atom revolve around the nucleus in circular paths.
- The size of the nucleus is very small as compared to the size of the atom.
Limitations of Rutherford’s Model:
- Accelerating electron releases energy, thus while moving electron will lose energy and thus is negatively charged will fall in the nucleus of positive charge.
- By this possession, the electron must be highly unstable, but in reality, atom is highly stable.
- This lead to the failure of his model. Read more in structure of the atom for class 9 notes.
Bohr’s Model of an Atom:
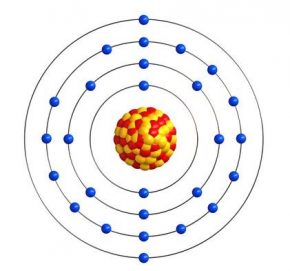
Assumptions made by Neil’s Bohr
- Inside the atom only certain special orbits known as discrete orbits of electrons, are allowed.
- While moving in that discrete orbits electrons do not lose its energy.
- These shells or obits are called energy levels.
- These shells are represented by letters K, L, M, N… or n=1, 2, 3, 4……
- Energy is absorbed or emitted when electrons transfer from one energy level to the other.
In the notes of Structure of the atom for Class 9 there is little explanation of Bohr’s model. Further you will read in higher sections.