(1) In the tetrahedral complex, [MX4]n±,
The metal atom or ion is placed at the centre of the regular tetrahedron and the four ligands, are placed at four corners of the tetrahedron.
(2) The ligands approach the central metal atom or ion in-between the three coordinates x, y and The orbitals dxy, dyz and d are pointed towards ligands and experience greater repulsion while the axial orbitals dx2-y2 and dz2 lie in-between metal-ligand bond axes and experience comparatively less experience.

Fig (a) and (b) : Tetrahedral geometry hasing central metal atoll) (M) at the centre and four ligands (L) al the four corners
(3) Therefore,
Energy of dxy, dyz and ddzx orbitals is increased while that of dx2y2 and dz2 is lowered. Hence 5d-orbitals lose their degeneracy and split into two point groups, namely f2? of higher energy (dxy, dyz and dzx) and e of lower energy (dx2-y2 and dz2).

Fig : Crystal field splitting in tetrahedral complex
(4) The experimental calculations show that the energy of t2g orbitals is increased by 0.4 Δ0 or 4Dq and energy of eg is lowered by 0.6 Δ0 or 6Dg.
Thus,
Energy difference between t2g and eg orbitals is Δ0 or 10Dg which is crystal field splitting energy (CFSE).
(5) This explains that the entry of each electron in eg orbitals,
Stabilises the complex by 0.6 Δ0 or 6Dq While the entry of each electron in t2g orbitals destabilises the tetrahedral complex by 0.4 Δ0 or 4Dg.
(6) In case of strong field ligands, the electrons prefer to pair up in eg orbitals giving low spin (LS) complexes while in case of weak held ligands, the electrons prefer to enter higher energy t2g orbitals giving more unpaired electrons and hence form high spin (HS) complexes.
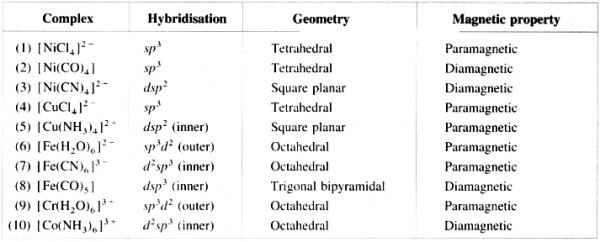
Table : Properties of complexes