The word "aromatic" through started with benzene and its derivatives only, now it signifies a large variety of organic compounds. To examine the presence of aromaticity, the following tips are useful. Ensure that your compound is cyclic. Each corner of the ring is either a double bonded atom, or must carry a negative charge or a positive charge or a hetero atom planar. You may get deceived while examining this. On the plane of the paper everything appears planar unless specially specified. Your compound should have a closed shell of (4n+2) electrons. When the closed loop contains 4n electrons, the system is rather less stable or antiaromatic. In fused ring system some of the rings give up their aromatic nature to adjacent rings in a property knwon as "annellation".
Which of the following is likely to be a solid
A.

B.
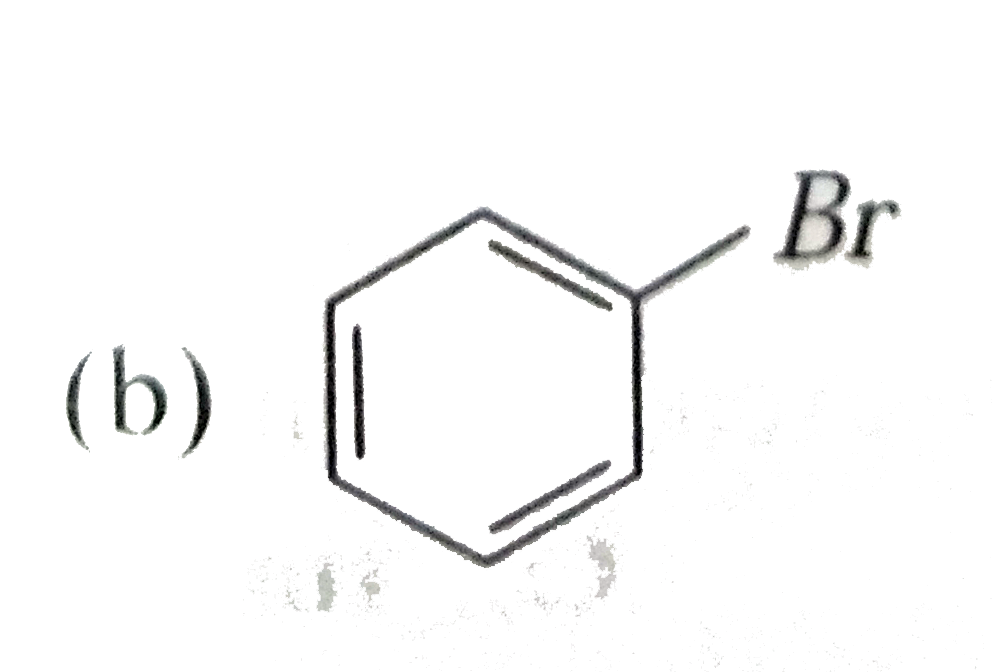
C.

D.
