An aqueous solution of metal ion `MI` reacts separately with reagents `Q` and `R` in excess to give tetrahedral and square planar complexes, respectively An aqueous solution of another metal ion `M2` always forms tetrahedral complexs with theses reagents. Aqueous solution of `M2` on reaction with reagent `S` gives white precipitate which dissolves in excess of `S` The reactions are summarised in the scheme given below: `SCHEME` :
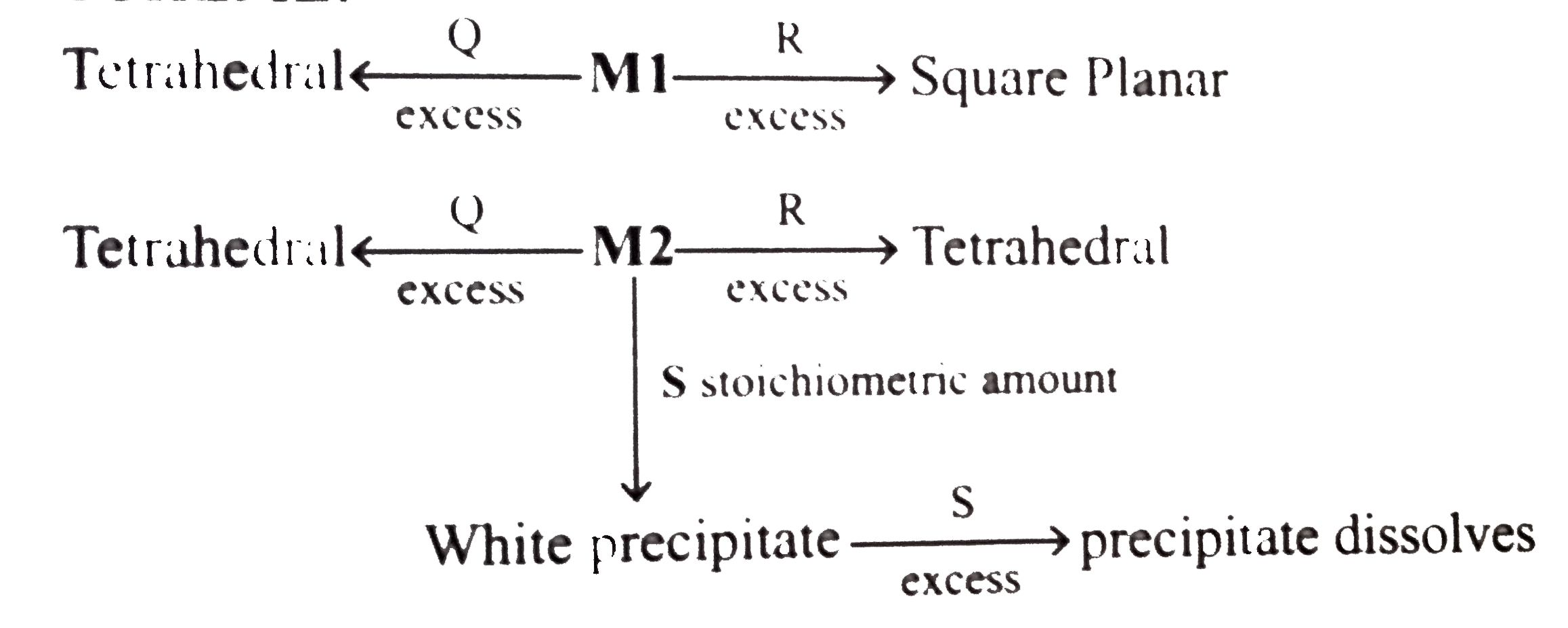
what is M2 and S??
A. `Zn^(2+)`, KCN, HCI and `K_(4)[Fe(CN)_(6)]`
B. `Ni^(2+)`, HCI, KCN and KOH
C. `Cd^(2+),KCN,HCl` and `Na_(2)HPO_(4)`
D. `Co^(2+)`, HCI, KCN and `K_(2)CrO_(4)`