Correct Answer - A
The methyl group decreases the acid strength of phenol from all positions i.e. all methylphenols (cresols) are weaker acids than phenol because the methyl group is electron donating inductivity from all positions and hyperconjugatively from the ortho and para positions.
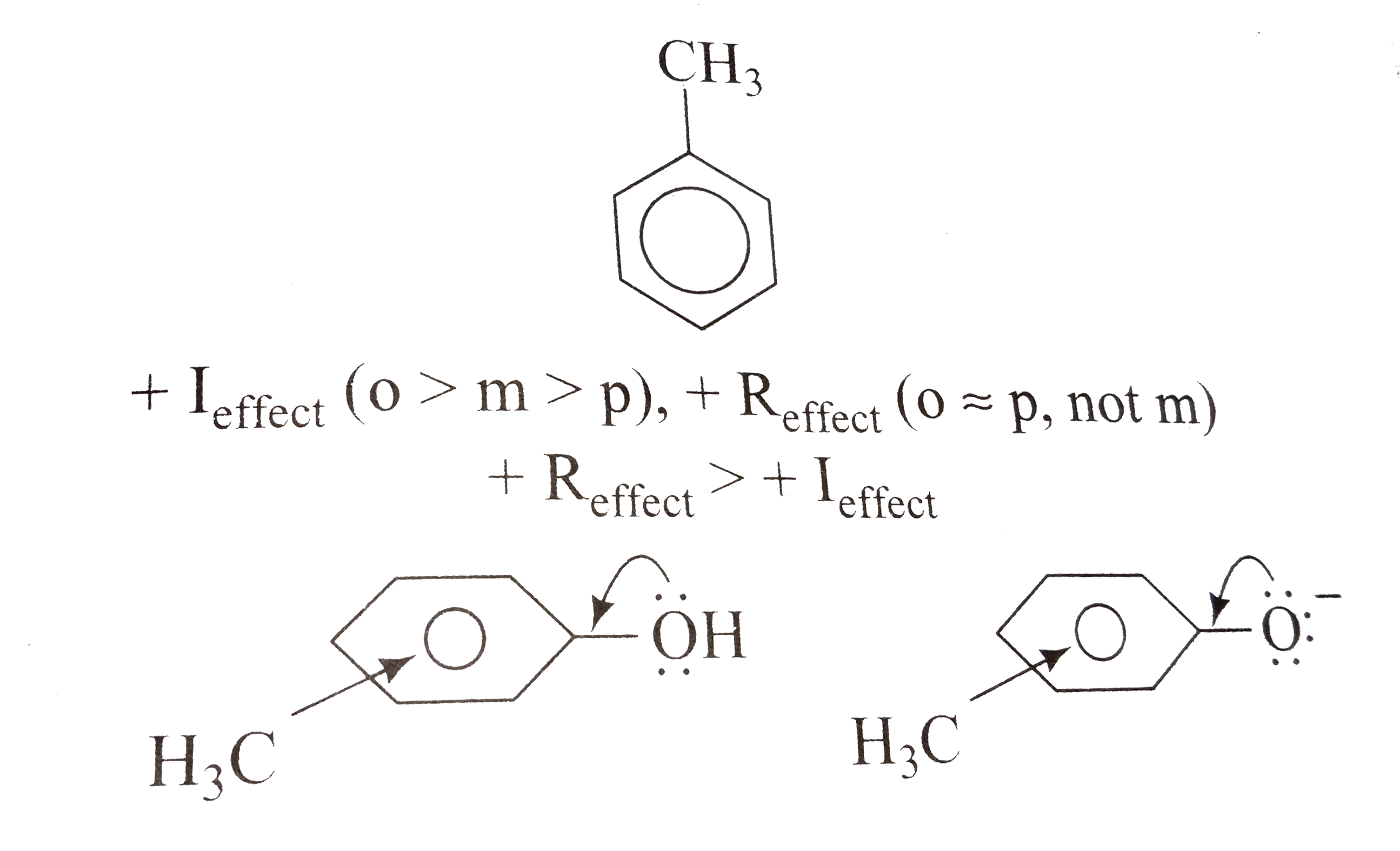
The methyl group is electron releasing (from all positions). Thus release of a lone pair from oxygen (of the `OH` group in the unionized phenol or from the `O^(-)` in the phenoxide ion) into the ring is opposed. This results in diminished resonance in the contributing structures.Consequently the phenoxide ion is more resonance stabilized with respect to phenol than is the methylphenoxide ion with respect to methyl phenol. Hence , phenol is a stronger acid than methylphenol.
`m`-Cresol is the strongest because its acidic strength is not weakened by hyperconjuction .`o`-Cresol is the weakest because in the `o`-position,`CH_(3)` group exerts the strongest `+ I_("effect")` . Thus , the decreasing order of acidic strength is
