Correct Answer - b
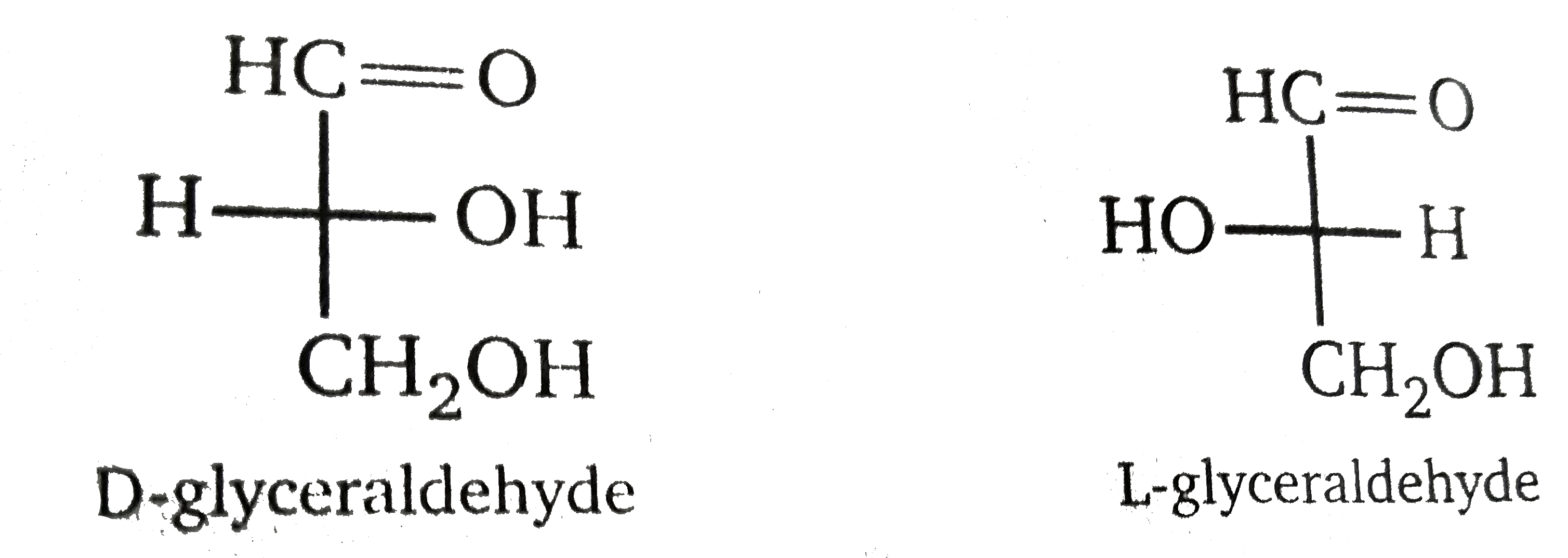
D and L notations can be used to describe the configuration of corbohydrates and amino acids, so it is important to learn what D and L. signify. In Fischer projections of monosaccharides, the carbonyl group is always placed on top ( in the case of aldoses) or as close to the top as possible (in hte case of ketoses). From its structure, you can see that galactose has four chirlity centers (C-2, C-3, C-4, and C-5). If the OH group attached to the bottom-most chiralirty center (the second from the left, the compound is and L-sugar. Almost all sugars found in nature are D-sugars. Notice that the mirror image of a D-sugar is an L-sugar.
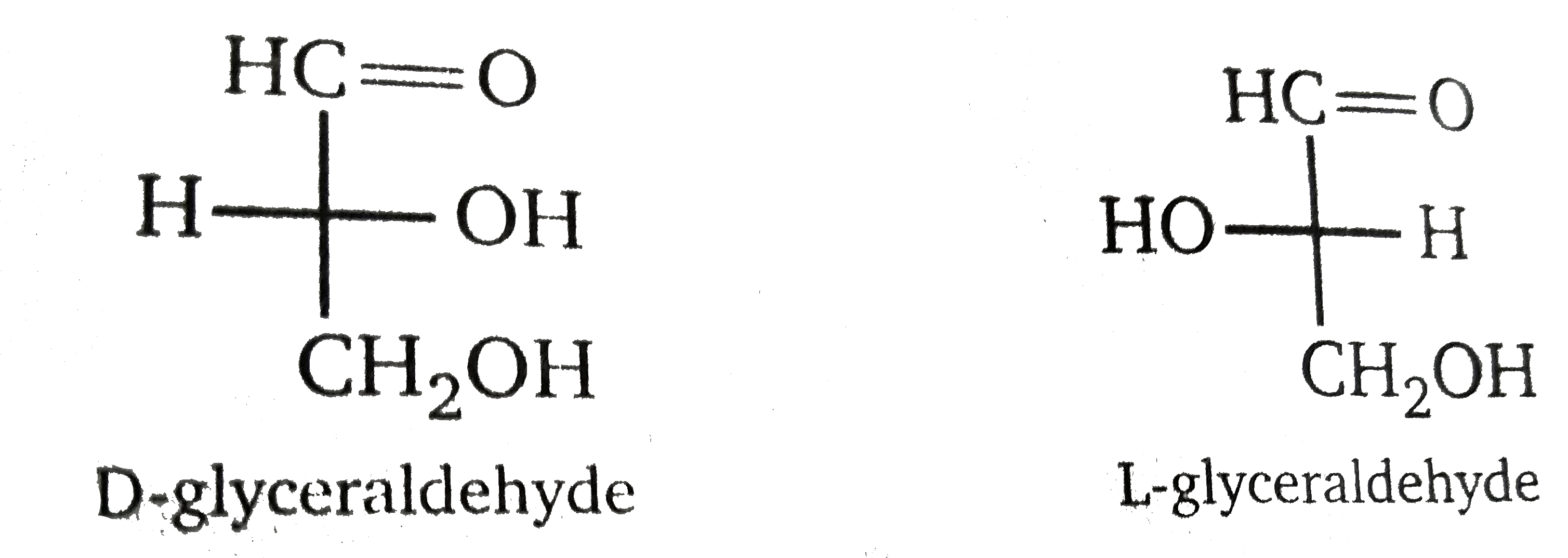
D nad L, like R and S, indicate the configuration of a chirality center, but they do not indicate whether the compound rotates plane-polarized light to the right (+) or levorotatory. In other words, optical rotation, like melting or boiling points, is a physical property of a compound, whereas "R,S, D and L" are convertions humans use to depict the structure of the molecule.
