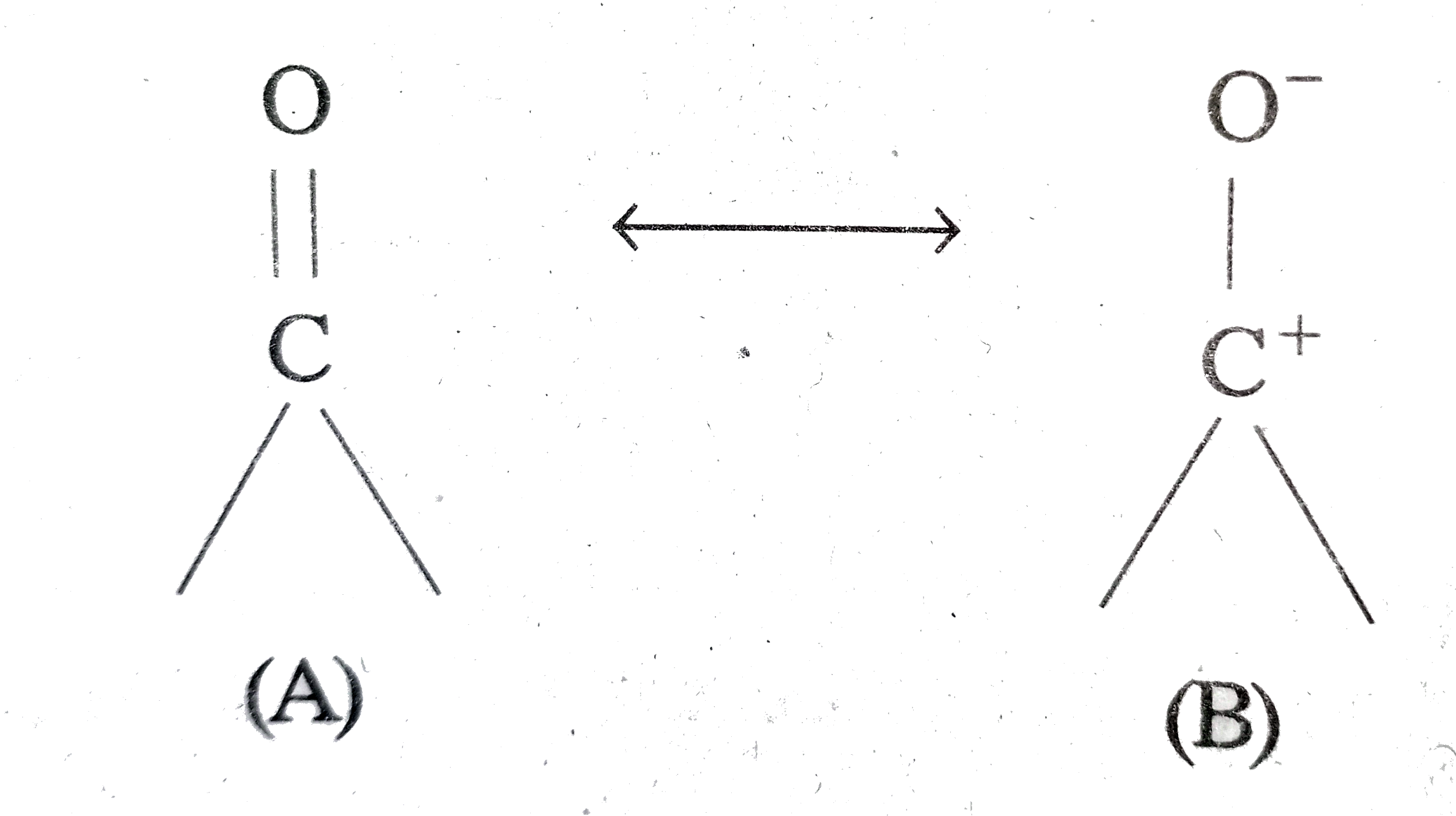
The carbon-oxygen double bond in carbonyl compounds is polarised due to higher electronegativity of oxygen relative to carbon. Hence the carbonyl carbon is an electrophilic and carbonxyl oxygen is a nucleophilic addition reaction.
`to` Alkenes contain

which is a source of electron density to electrophilies add no to C =C to give addition products. Hence alkenes undergo electrophilic addition reaction.