Precision :
| Measurement |
Mass of acetone observed (g) |
| 1 |
38.7798 – 38.0015 = 0.7783 |
| 2 |
38.7795 – 38.0015 = 0.7780 |
| 3 |
38.7801 – 38.0015 = 0.7786 |
Mean = \(\frac{0.7783+0.7780+0.7786}{3}\)
= 0.7783 g
| Measurement |
Mass of acetone observed (g) |
Absolute deviation (g) = | Observed value – Mean | |
| 1 |
0.7783 |
0 |
| 2 |
0.7780 |
0.0003 |
| 3 |
0.7786 |
0.0003 |
Mean absolute deviation = \(\frac{0+0.0003+0.0003}{3}\)
= 0.0002
∴ Mean absolute deviation = ±0.0002 g
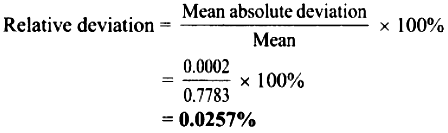
Accuracy :
Actual mass of acetone = 0.7791 g
Observed value (average) = 0.7783 g
a. Absolute error = Observed value – True value
= 0.7783 – 0.7791
= – 0.0008 g

These observed values are close to each other and are also close to the actual mass.
Therefore,
The results are precise and as well accurate.
i. Relative deviation = 0.0257%
ii. Relative error = 0.1027%
[Note : i. As per the method given in textbook, the calculated value of relative deviation is 0.0257%.
ii. The negative sign in -0.1027% indicates that the experimental result is lower than the true value.]