Correct Answer - A
`._(28)Ni=1s^(2), 2s^(2)2p^(6), 3s^(2)3p^(6)3d^(8),4s^(2)`
`Ni^(2+)=1s^(2), 2s^(2)2p^(6),3s^(2)3p^(6)3d^(8)`
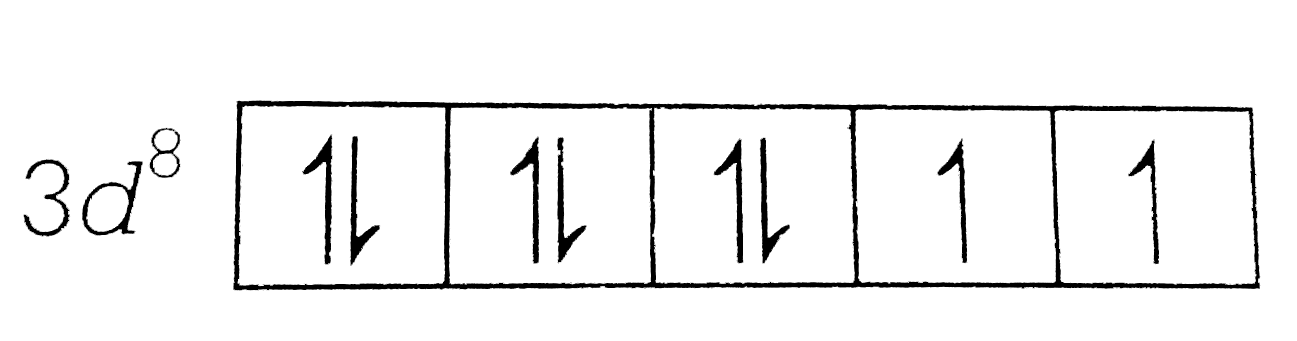
`._(22)Ti=1s^(2), 2s^(2)2p^(6), 3s^(2)3p^(6)3d^(2),4s^(2)`
`Ti^(3+) = 1s^(2), 2s^(2)2p^(6), 3s^(2)3p^(6)3d^(1)` (1 unpaired electron)
`._(21)Sc=1s^(2),2s^(2)2p^(6),3s^(2)3p^(6)3d^(1),4s^(2)`
`Sc^(3+) = 1s^(2), 2s^(2)2p^(6),3s^(2)3p^(6)` (no unpaired electron)
`._(29)Cu= 1s^(2), 2s^(2)2p^(6), 3s^(2)3p^(6)3d^(10),4s^(1)`
`Cu^(+) = 1s^(2), 2s^(2)2p^(6),3s^(2)3p^(6)3d^(10)` (no unpaired electron)
Hence, in the above ions `Ni^(2+)`and `Ti^(3+)` are coloured in aqueous solution due to the presence of unpaired electrons in d subshell.