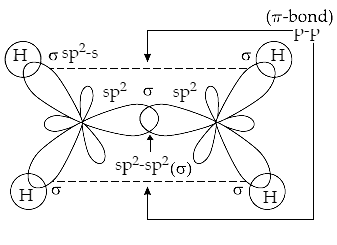
Structure of C2H4 on the basis of Hybridisation: Carbon – carbon double bond in ethene (C2H4) consists of one s bond due to head-on overlapping of sp2 hybridised orbitals and one π - bond obtained by sideways overlapping of the two 2p orbitals of the two carbon atoms. The structure of C2H4 is shown as below –