a. Ethanol; C2 H5 OH
Ethanol is a colourless liquid having boiling point 351k and melting point 156k
Also it is soluble in water
Its formula is C2 H5 OH
Electron Dot Structure of Ethanol is as follows
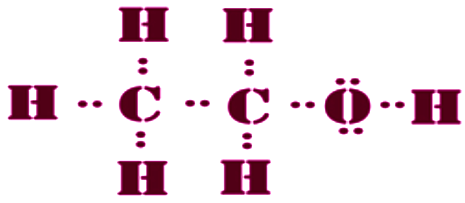
(b) Ethanol is an alcohol
Examples of Alcohols
|
|
CH3 OH |
| Ethanol |
C2 H5 OH |
| Propanol |
C3 H7 OH |
| Butanol |
C4 H9 OH |
Note:
All the above are homologues
Homologues are group of chemical compounds which have similar structure and similar chemical properties and successive compounds differ by CH2
We can say that homologues of ethanol are