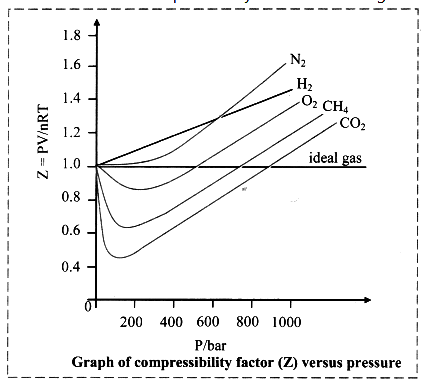
Compressibility factor (Z):
i. It is defined as the ratio of product PV and nRT.
z = \(\frac{PV}{nRT}\)
ii. Deviation from ideal behaviour is measured in terms of compressibility factor.
iii. For ideal gases, Z = 1 under all conditions of temperature and pressure. Therefore, the graph of Z versus P will be a straight line parallel to pressure axis.
iv. For gases that deviate from ideal behaviour, value of Z deviates from unity. Note: Variation of compressibility factor for some gases