The structural formulae of two reagents showing all the valence electrons are:

Thus, NH3 contains N with a lone pair of electrons which can be given away to another species. Therefore, NH3 is a nucleophile and ‘N’ in it is the nucleophilic centre.
The 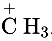 is a positively charged electron deficient species having a vacant orbital on the carbon. It is an electrophile and the ‘C’ in it is the electrophilic centre.
is a positively charged electron deficient species having a vacant orbital on the carbon. It is an electrophile and the ‘C’ in it is the electrophilic centre.