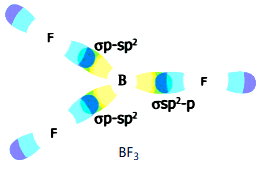
Formation of BF3 molecule :
1. 5B has electronic configuration 1s2 2s2 2px .
2. The excited electronic configuration of 5B is 1s2 2s1 2px2 2py2.
3. As it forms three identical B-F bonds in BF3
4. It is suggested that excited ‘B’ atom undergoes hybridisation.
5. There is an intermixing of 2s, 2px , 2py orbitals and their redistribution into three identical orbitals called sp2 hybrid orbitals.
6. For three sp2 orbitals to get separated to have minimum repulsion the angle between any two orbitals is 120° at the central atom and each sp2 orbital gets one electron.
7. Now three fluorine atoms overlap their 2pz orbitals containing unpaired electrons (F9 1s2 2s2 2px2 2py2 2px2) the three sp2 orbitals of ‘B’ that contain unpaired electrons to form three ssp2-p bonds.