Copper sulphate solution is electrolysed using copper electrodes. Study the diagram given below and answer the question that follows:
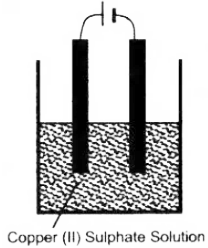
(i) Which electrode to your left or right is known as the oxidising electrode and why ?
(ii) Write the equation representing the reaction that occurs.
(iii) State two appropriate observations for the above electrolysis reaction.