14. In the alkane H3C–CH2–C(CH3)2–CH2–CH(CH3)2, identify 1°,2°,3° carbon atoms and give the number of H atoms bonded to each one of these.
Answer:

1° carbon atoms are those which are bonded to only one carbon atom i.e., they have only one carbon atom as their neighbour. The given structure has five 1° carbon atoms and fifteen hydrogen atoms attached to it.
2° carbon atoms are those which are bonded to two carbon atoms i.e., they have two carbon atoms as their neighbours. The given structure has two 2° carbon atoms and four hydrogen atoms attached to it.
3° carbon atoms are those which are bonded to three carbon atoms i.e., they have three carbon atoms as their neighbours. The given structure has one 3° carbon atom and only one hydrogen atom is attached to it.
15. What effect does branching of an alkane chain has on its boiling point?
Answer:
Alkanes experience inter-molecular Van der Waals forces. The stronger the force, the greater will be the boiling point of the alkane. As branching increases, the surface area of the molecule decreases which results in a small area of contact. As a result, the Van der Waals force also decreases which can be overcome at a relatively lower temperature. Hence, the boiling point of an alkane chain decreases with an increase in branching.
16. Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer:
Addition of HBr to propene is an example of an electrophilic substitution reaction.
Hydrogen bromide provides an electrophile, H+. This electrophile attacks the double bond to form 1° and 2° carbocations as shown:

Secondary carbocations are more stable than primary carbocations. Hence, the former predominates since it will form at a faster rate. Thus, in the next step, Br– attacks the carbocation to form 2 – bromopropane as the major product.

This reaction follows Markovnikov’s rule where the negative part of the addendum is attached to the carbon atom having a lesser number of hydrogen atoms. In the presence of benzoyl peroxide, an addition reaction takes place anti to Markovnikov’s rule. The reaction follows a free radical chain mechanism as:

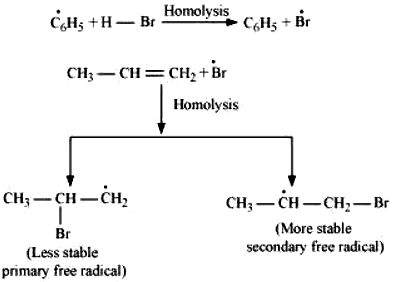
Secondary free radicals are more stable than primary radicals. Hence, the former predominates since it forms at a faster rate. Thus, 1 – bromopropane is obtained as the major product.

In the presence of peroxide, Br free radical acts as an electrophile. Hence, two different products are obtained on addition of HBr to propene in the absence and presence of peroxide.
17. Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekulé structure for benzene?
Answer:
o-xylene has two resonance structures:

All three products, i.e., methyl glyoxal, 1, 2-demethylglyoxal, and glyoxal are obtained from two Kekule structures. Since all three products cannot be obtained from any one of the two structures, this proves that o-xylene is a resonance hybrid of two Kekule structures (I and II).
18. Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Answer:
Acidic character of a species is defined on the basis of ease with which it can lose its H– atoms.
The hybridization state of carbon in the given compound is:

As the s–character increases, the electronegativity of carbon increases and the electrons of C–H bond pair lie closer to the carbon atom. As a result, partial positive charge of H– atom increases and H+ ions are set free.
The s–character increases in the order:
sp3 < sp2 < sp
Hence, the decreasing order of acidic behaviour is Ethyne > Benzene > Hexane.
19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer:
Benzene is a planar molecule having delocalized electrons above and below the plane of ring. Hence, it is electron-rich. As a result, it is highly attractive to electron deficient species i.e., electrophiles.
Therefore, it undergoes electrophilic substitution reactions very easily. Nucleophiles are electron-rich. Hence, they are repelled by benzene. Hence, benzene undergoes nucleophilic substitutions with difficulty.
20. How would you convert the following compounds into benzene?
(i) Ethyne
(ii) Ethene
(iii) Hexane
Answer:
(i) Benzene from Ethyne:

(ii) Benzene from Ethene:

(iii) Hexane to Benzene

21. Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer:
The basic skeleton of 2-methylbutane is shown below:

On the basis of this structure, various alkenes that will give 2-methylbutane on hydrogenation are:
(a)

(b)

(c)

22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p-H3C–C6H4–NO2, p-O2N–C6H4–NO2.
Answer:
Electrophiles are reagents that participate in a reaction by accepting an electron pair in order to bond to nucleophiles.
The higher the electron density on a benzene ring, the more reactive is the compound towards an electrophile, E+ (Electrophilic reaction).
(a) The presence of an electron withdrawing group (i.e., NO2–and Cl–) deactivates the aromatic ring by decreasing the electron density.
Since NO2– group is more electron withdrawing (due to resonance effect) than the Cl– group (due to inductive effect), the decreasing order of reactivity is as follows:
Chlorobenzene > p – nitrochlorobenzene > 2, 4 – dinitrochlorobenzene
(b) While CH3– is an electron donating group, NO2– group is electron withdrawing. Hence, toluene will have the maximum electron density and is most easily attacked by E+. NO2– is an electron withdrawing group. Hence, when the number of NO2– substituents is greater, the order is as follows:
Toluene > p–CH3–C6H4–NO2, p –O2 N–C6H4–NO2