Heat supplied at 290°C = 300 kJ/s
Heat rejected at 8.5°C : (i) 215 kJ/s, (ii) 150 kJ/s, (iii) 75 kJ/s.
Applying Clausius inequality to the cycle or process, we have :
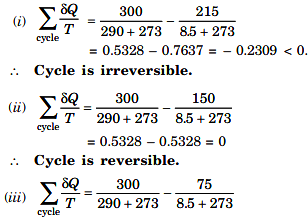
= 0.5328 – 0.2664 = 0.2664 > 0.
This cycle is impossible by second law of thermodynamics, i.e., Clausius inequality.