
In it Xe is sp3 d-hybridised but its shape is linear due to involvement of VSEPR theory. (ie, due to presence of three free pair of electrons, geometry of XeF2 is distorted from trigonal bipyramidal to linear)

trn it Xe is sp3 d2-hybridised but its shape is square planar due to involvement of VSEPR theory. (ie, due to presence of two free pair of electrons, geometry of XeF4 is distorted from octahedral to square planar).
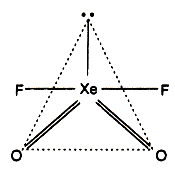
In it Xe is sp3 d-hybrid, but its geometry is Planar due to involvement of VSEPR theory. (ie, due to presence of a free pair of electrons, its geometry is distorted from trigonal bipyramidal to planar.)