NCERT Solutions Class 12 Chemistry Chapter 3 Electrochemistry
1. Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn
Answer:
The following is the order in which the given metals displace each other from the solution of their salts.
Mg, Al, Zn, Fe, Cu
2. Given the standard electrode potentials,
K+/K = −2.93V, Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = −2.37 V, Cr3+/Cr = − 0.74V
Arrange these metals in their increasing order of reducing power.
Answer:
The lower the reduction potential, the higher is the reducing power. T
he given standard electrode potentials increase in the order of K+/K < Mg2+/Mg < Cr3+/Cr < Hg2+/Hg < Ag+/Ag.
Hence, the reducing power of the given metals increases in the following order:
Ag < Hg < Cr < Mg < K
3. Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Answer:
The galvanic cell in which the given reaction takes place is depicted as:

(i) Zn electrode (anode) is negatively charged.
(ii) Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
(iii) The reaction taking place at the anode is given by,
Zn(s) \(\longrightarrow\) Zn2+(aq) + 2e-
The reaction taking place at the cathode is given by,
Ag+(aq) + e- \(\longrightarrow\) Ag(s)
4. Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ∆rGθ and equilibrium constant of the reactions.
Answer:
(i) \(E^\Theta _{cr^{3+}/cr} = 0.74 \,V\)
\(E^\Theta _{cd^{2+}/c4} = -0.4 0\,V\)
The galvanic cell of the given reaction is depicted as:

Now, the standard cell potential is

In the given equation,
n = 6
F = 96487 C mol−1
\(E^{\Theta}_{cell}\) = +0.34 V
Then, \(\Delta _rG^\Theta\) = −6 × 96487 C mol−1 × 0.34 V
= −196833.48 CV mol−1
= −196833.48 J mol−1
= −196.83 kJ mol−1
Again,
\(\Delta _rG^\Theta\) = −RT ln K

= 34.496
\(\therefore\) K = antilog (34.496) = 3.13 × 1034
(ii) \(E^\Theta_{Fe^{3+}/Fe^{2+}} = 0.77\,V\)
\(E^\Theta_{Ag^+/Ag}= 0.80\,V\)
The galvanic cell of the given reaction is depicted as:

Now, the standard cell potential is

Here, n = 1.
Then,
\(\Delta _rG^\Theta = -nFE^\Theta_{cell}\)
= −1 × 96487 C mol−1 × 0.03 V
= −2894.61 J mol−1
= −2.89 kJ mol−1
Again,

= 0.5073
\(\therefore\) K = antilog (0.5073)
= 3.2 (approximately)
5. Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
(ii) Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
(iii) Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
(iv) Pt(s) | Br2(l) | Br−(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
Answer:
(i) For the given reaction, the Nernst equation can be given as:
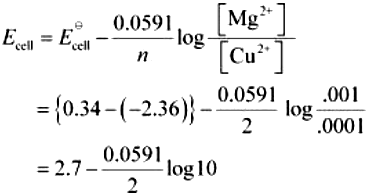
= 2.7 − 0.02955
= 2.67 V (approximately)
(ii) For the given reaction, the Nernst equation can be given as:

= 0.52865 V
= 0.53 V (approximately)
(iii) For the given reaction, the Nernst equation can be given as:

= 0.14 − 0.0295 × log125
= 0.14 − 0.062 = 0.078 V
= 0.08 V (approximately)
(iv) For the given reaction, the Nernst equation can be given as:

6. In the button cells widely used in watches and other devices the following reaction takes place:

Determine \(\Delta _rG^\Theta\) and \(E^{\Theta}\) for the reaction.
Answer:
\(E^{\Theta}\) = 1.104 V
We know that,
\(\Delta _rG^\Theta= -nFE^\Theta\)
= −2 × 96487 × 1.04
= −213043.296 J
= −213.04 kJ