15. What will be the mechanism for the following reaction?

Answer:
The given reaction is

CN– acts as the nuclephile and attacks the carbon atom on which the Br is attached. CN– ion is an ambient nucleophile and can attack through both C and N positions. It attacks through C atom, in this case.

16. Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2- methylbutane
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1Bromo-3- methylbutane.
Answer:
(i)

An SN2 reaction involves the approaching of the nucleophile to the carbon atom to which the leaving group is attached. When the nucleophile is sterically hindered, then the reactivity towards SN2 displacement decreases. Due to the presence of substituents, hindrance to the approaching nucleophile increases in the following order.
1-Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane
Hence, the increasing order of reactivity towards SN2 displacement is:
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
(ii)

Since steric hindrance in alkyl halides increases in the order of 1° < 2° < 3°, the increasing order of reactivity towards SN2 displacement is 3° < 2° < 1°.
Hence, the given set of compounds can be arranged in the increasing order of their reactivity towards SN2 displacement as:
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
(iii)

The steric hindrance to the nucleophile in the SN2 mechanism increases with a decrease in the distance of the substituents from the atom containing the leaving group. Further, the steric hindrance increases with an increase in the number of substituents. Therefore, the increasing order of steric hindrances in the given compounds is as below:
1-Bromobutane < 1-Bromo-3-methylbutane < 1-Bromo-2-methylbutane < 1-Bromo-2, 2-dimethylpropane
Hence, the increasing order of reactivity of the given compounds towards SN2 displacement is:
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1-Bromobutane
17. Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH?
Answer:

Hydrolysis by aqueous KOH proceeds through the formation of carbocation. If carbocation is stable, then the compound is easily hydrolyzed by aqueous KOH. Now,
C6H5CH2Cl forms 1o– carbocation, while C6H5CHClC6H5 forms 2o– carbocation, which is more stable than 1o– carbcation. Hence, C6H5CHClC6H5 is hydrolyzed more easily than C6H5CH2Cl by aqueous KOH.
18. p-Dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss.
Answer:

p-Dichlorobenzene is more symmetrical than o-and m-isomers. For this reason, it fits more closely than o-and m-isomers in the crystal lattice. Therefore, more energy is required to break the crystal lattice of p-dichlorobenzene. As a result, p-dichlorobenzene has a higher melting point and lower solubility than o-and m-isomers.
19. How the following conversions can be carried out?
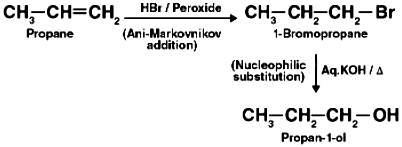
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
Answer:
(i)






20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Answer:
In an aqueous solution, KOH almost completely ionizes to give OH− ions. OH− ion is a strong nucleophile, which leads the alkyl chloride to undergo a substitution reaction to form alcohol.

On the other hand, an alcoholic solution of KOH contains alkoxide (RO−) ion, which is a strong base. Thus, it can abstract a hydrogen from the β-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.

H− ion is a much weaker base than RO− ion. Also, OH− ion is highly solvated in an aqueous solution and as a result, the basic character of OH− ion decreases. Therefore, it cannot abstract a hydrogen from the β-carbon.