11. An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
Answer:
A is an organic compound with a molecular formula C8H16O2. This gives a carboxylic acid (B) and alcohol (C) on hydrolysis with dilute sulphuric acid. Thus, compound A must be an ester.
Further, oxidation of alcohol (C) with chromic acid gives acid B. Thus, B and C must contain an equal number of carbon atoms.
A total of 8 carbon atoms are present in compound A, each of B and C contain 4 carbon atoms.
Again, alcohol C gives but-1-ene on dehydration. Therefore, C is of straight-chain and hence, it is butan-1-ol.
On oxidation, Butan-1-ol gives butanoic acid. Hence, acid B is butanoic acid.
Hence, the ester with molecular formula C8H16O2 is butylbutanoate.

All the given reactions can be explained by the following equations.

12. Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
(i) When HCN reacts with a compound, the attacking species is a nucleophile, CN–. Therefore, the reactivity with HCN decreases, when the negative charge on the compound increases. The +I affect increases in the given compound. Steric hindrance also increases in the same.
Hence, the given compounds can be arranged according to their increasing reactivities toward HCN as:

Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde
(ii) After losing a proton, carboxylic acids gain a negative charge as shown:

Now, the stability of the carboxyl ion increases by any group that helps to stabilize the negative charge, will increase the strength of the acid. Thus, groups having −I effect will increase the strength of the acids and groups having +I effect will decrease the strength of the acids. In the given compounds,Br− group has −I effect and −CH3 group has +I effect. Thus, acids containing Br− are stronger.
Now, the +I effect of isopropyl group is more than that of n-propyl group. Hence, CH3CH2CH2COOH is a stronger acid than (CH3)2CHCOOH.
Also, as the distance increases, +I effect grows weaker. Hence, CH3CH2CH(Br)COOH is a stronger acid than CH3CH(Br)CH2COOH.
Hence, the strengths of the given acids increase as:
(CH3)2CHCOOH < CH3CH2CH2COOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
(iii) As we have seen in the previous case, the strengths of acids is decreased by the electron donating group, while the strengths of acids increases by electron-withdrawing groups. As methoxy group is an electron-donating group, benzoic acid is a stronger acid than 4-methoxybenzoic acid. Nitro group is an electron-withdrawing group and will increase the strengths of acids. As 3,4-dinitrobenzoic acid contains two nitro groups, it is a slightly stronger acid than 4-nitrobenzoic acid. Hence, the strengths of the given acids increase as:
4-Methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3, 4-Dinitrobenzoic acid
13. Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
Answer:
(i) Propanal and propanone can be distinguished by the following tests.
(a) Tollen’s test
Tollen’s reagent is reduced by propanal as it is an aldehyde. But, Tollen’s reagent is not reduced as propanone is a ketone.

(b) Fehling’s test
Aldehydes respond to Fehling’s test, but ketones do not.
Propanone being a ketone does not reduce Fehling’s solution to a red-brown precipitate of Cu2O, but Propanal being an aldehyde does the reaction.

(c) Iodoform test:
At least one methyl group should be present in aldehydes and ketones linked to the carbonyl carbon atom to respond to iodoform test. They are oxidized by sodium hypoiodite (NaOI) to give iodoforms. Propanone being a methyl ketone responds to this test, but propanal does not.

(ii) Acetophenone and Benzophenone can be distinguished using the iodoform test.
Iodoform test:
yellow ppt. of iodoform is produced when methyl ketones are oxidized by sodium hypoiodite.
Acetophenone being a methyl ketone responds to this test, but benzophenone does not.

(iii) Phenol and benzoic acid can be distinguished by ferric chloride test.
Ferric chloride test:
Phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet coloration.
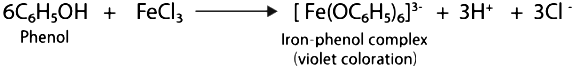
A buff coloured ppt of ferric benzoate is produced when benzoic acid reacts with neutral FeCl3.

(iv) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test.
Sodium bicarbonate test:
Brisk effervescence is produced when acids react with NaHCO3 due to the evolution of CO2 gas.
Benzoic acid being an acid responds to this test, but ethylbenzoate does not.
