(i) Oxidation number of Fe
= x + 2 x 0 + 2(-1)
= +1
x – 2 = +1
⇒ x = 3
(ii) Orbitals in Fe+3(d5)
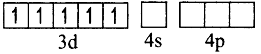
In the presence of a strong ligand (en), the electrons in 3d orbital get paired.
Fe+3 excited state
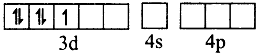
Thus, Fe+3 ion undergoes d2sp3 hybridisation therefore the shape is octahedral.
(iii) The complex is paramagnetic due to the presence of one unpaired electron.
(iv) It exhibits cis-trans isomerism
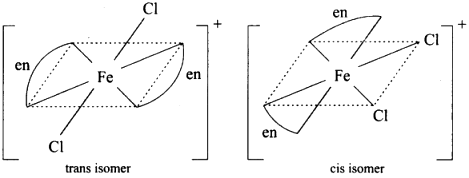
(v) Cis isomer will show optical isomerism.

(vi) Dichlorido bis(ethane 1, 2, diamine) iron, (III) chloride.