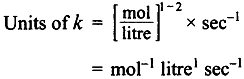(a) Order with respect to NO and Br2 are 2 and 1 respectively, over all order is 3.

(b) Order 3/2

(c) Order with respect to H2 and NO are 1 and 2 respectively.

= mol-2 litre2 sec-1
(d) Order with respect to CO and Cl2 are 2 and \(\frac 12\) respectively.
Over all order = \(2\frac 12 = \frac 52\)
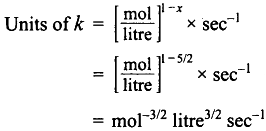
(e) Order with respect to H2O2 and I– are one respectively. Over all order 1 + 1 = 2