Given :
pH = 11.11;
percent Dissociation of base = ?
c = 0.1 M
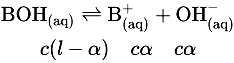
pH + pOH = 14
∴ pOH = 14 – pH = 14 – 11.11 = 2.89
POH = -log10[OH-]
∴ [OH-] = Antilog – pOH
= Antilog – 2.89
= Antilog \(\bar 3.11\)
= 1.29 x 10-3 M
∵ [OH-] = cα
∴ α = \(\frac{[OH^-]}{c}\)
= \(\frac{1.29 \times 10^{-3}}{0.1}\)
= 1.29 x 10-2
∴ Percent dissociation = α × 100
= 1.29 × 10-2 × 100
= 1.29
∴ Percent dissociation = 1.29