MO2 & M2O3
Approximate atomic mass = 6.4 / 0.117 = 54.7
For first oxide 63.2 g metal combines with 36.8 g oxygen 54.7 g metal combines with
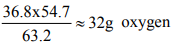
So 1 mole atom of metal combines with 2 mole atoms of O Formula is MO2
For second oxide 69.62 g metal combines with 30.38 g oxygen
54.7 g metal combines with
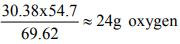
So 1 mole atom of metal combines with 1.5 mole atoms of O Formula is M2O3