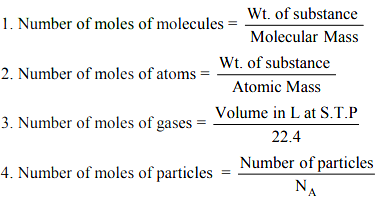
5. If volume of gas is given along with its temperature (T) & pressure (P) then n = PV/RT
where R = 0.0821 lit-atm/mol K & P is in atmosphere, T in Kelvin and V in litre
Note:
1. Do not use the above expression for solids/liquids for eg. H2O at 10 °C.
2. 1 gm - atom is same as 1 mole of an atom & hence will have weight equal to atomic weight expressed in grams.
3. 1 gm – molecule is same as 1 mole of the molecule & hence will have weight equal to molecular weight expressed in gms.
4. 1 gm – Ion is same as 1 mole of an ion & hence will have weight equal to ionic weight
5. Remember 1 gm of atom & 1 gm– atom are two different phrases. Former is mentioning weight (equal to 1 gm) & latter is mentioning moles.
For Example:
(1) x g atom of nitrogen = x moles of N atom = X x NA number of N atoms
(2) x g molecule of nitrogen = x moles of N2 molecules = X x NA molecules of N2 = 2x x NA number of N atoms.