(i) Mole of oxygen = 448 / 22400 = 0.02
Wt. of oxygen = 0.02 x 32 = 0.64 gm

Applying POAC on O atoms
Moles of O atoms in KClO3 = moles of O atoms in O2
3 (moles of KClO3) = 2 (moles of O2)
(1 mole of KClO3 contains 3 moles of O and 1 mole of O2 contains 2 moles of O)
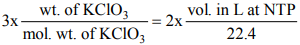
Wt. of KClO3 = 1.634 g
(iii) Again applying POAC for K atoms,
Moles of K atoms in KClO3 = 1 x moles of KCl
(1 mole of KClO3 contains 1 mole of K and 1 mole of KCl contains 1 mole of K)
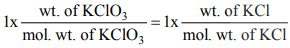
Wt. of KCl = 0.9937 g.