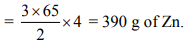The given equations are
Zn + 2HCl → ZnCl2 + H2
3H2 + N2 → 2NH3
From the equations it is clear that
2 mol of NH3 require = 3 mol of H2 ;
3 mol of H2 require = 3 mol of Zn Thus, 2 mol of NH3 require = 3 mol of Zn = 3 × 65 g of Zn
∴ 4 mol of NH3 require