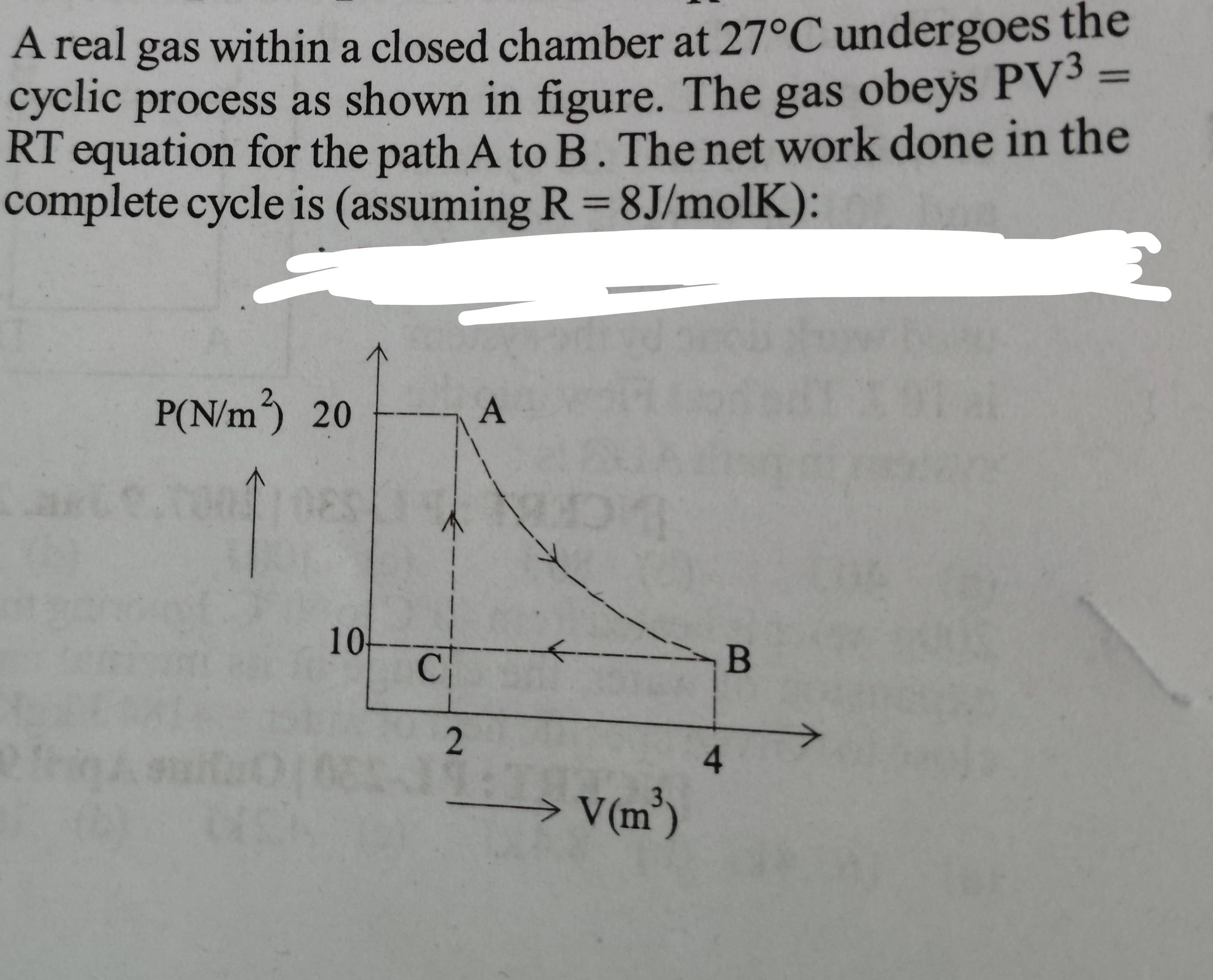
A real gas within a closed chamber at \( 27^{\circ} C \) undergoes the cyclic process as shown in figure. The gas obeys \( PV ^{3}= \) RT equation for the path \( A \) to \( B \). The net work done in the complete cycle is (assuming \( R =8 J / molK \) ):
solve by work done in polytropic process formula for path Ato B