Given below are two statements:
Consider the following reaction

Statement (I) : In the case of formaldehyde

K is about 2280, due to small substituents, hydration is faster.
Statement (II) : In the case of trichloro acetaldehyde
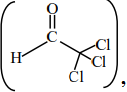
K is about 2000 due to – I effect of – Cl.
In the light of the above statements, choose the correct answer from the options given below:
(1) Statement I true but Statement II is false
(2) Both Statement I and Statement II are true
(3) Statement I is false but Statement II is true
(4) Both Statement I and Statement II are false