Molecular orbital energy level diagram (MOED) of 'N2' :
Electronic configuration of nitrogen (Z = 7) is 1s2 2s2 2p3. Since nitrogen atom has 7 electrons, the molecular orbitals of nitrogen molecule (N2) has 14 electrons which are distributed as below :


Molecular orbital energy level diagram of N2 molecule
• Bond order = (8 2)/2 = 3 (N ≡ N)
• Absence of unpaired electrons showed that N2 molecule is diamagnetic.
MOED of 'O2' :
Electronic configuration of Oxygen (Z = 8) is 1s2 2s2 2p4 . Since Oxygen atom has 8 electrons, the molecular orbitals of Oxygen molecule (O2) has 16 electrons, which are distributed as below :
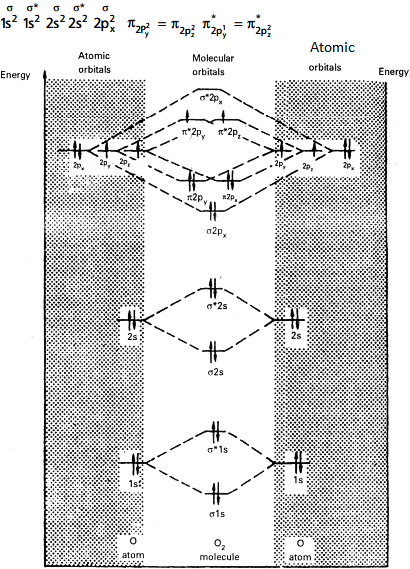
Molecular orbital energy level diagram of O2
• Bond order = (10 - 6)/2 = 2(O = O)
• Presence of two unpaired 6 electrons (π* 2p1y π*2p1z,) showed that O2 molecule is paramagnetic.