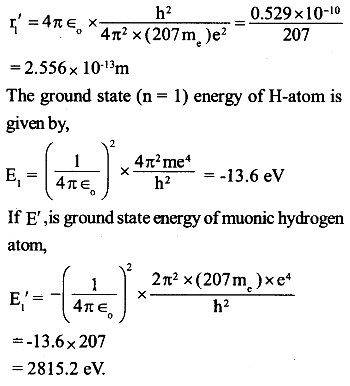
Let m be the mass of the electron the first Bohr radius of H-atom is given by,

in a muonic hydrogen atom, in place of an electron, a negatively charged muon having mass 207me. revolves around the nucleus of hydrogen i.e. proton.
If r1‘ is first Bohr’s radius of muonic hydrogen atom,