1.
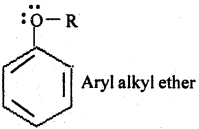
In aryl alkyl ethers, due to +R effect of the alkoxy group, the electron density in the benzene ring increases as shown in the following resonance structure

Thus, benzene is activated towards electrophilic substitution by the alkoxy group.
2. It can be observed from the resonance structures that the electron density increases more at the ortho and para positions that at the meta position. As a result, the incoming substituents are directed to the ortho and para positions in the benzene ring.