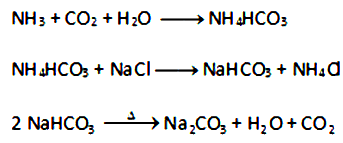• Washing soda is sodium carbonate containing 10 molecules of water of crystallisation.
• It is called as sodium carbonate decahydrate. It is prepared by Solvay's process.
• A cold and concentrated solution of sodium chloride is reacted with ammonia and carbon dioxide to obtain sodium hydrogen carbonate.
• It can remove temporary and permanent hardness from water. Sodium carbonate is soluble but calcium carbonate and magnesium carbonate are insoluble.
• The water is softened because it no longer contains dissolved calcium ions and magnesium ions.
• It will form lather more easily with soap