Solubility Product: It is defined as the product of the concentration of ions in a saturated solution of an electrolyte at a given temperature. It is repersented by the symbol Ksp.
Relation between solubility and solubility product for CdS type compounds
Let x be the solubility of CdS
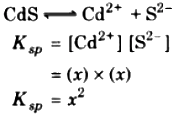
In third group analysis, NH4 Cl is added before NH4 OH because NH+4 ions furnished by NH4Cl lower the ionization of NH4 OH and hence the concentration of OH– ion. At low concentration of OH– ion, III group hydroxides precipitate.
Whereas, when NH4OH is added in the presence of NH4Cl, the precipitation of group hydroxides takes place.