(i) Zeolites:- zeolites are microporous crystalline solids with well-defined structures. Generally, they contain silicon, aluminium and oxygen in their framework and cations, water and/or other molecules within their pores.
Zeolite is the broad term used to describe a family of minerals called tectosilicates. These minerals contain small pores which provide a general surface area. These molecule contain tetrahedral AlO5-4 and SiO4-4 molecules bound by oxygen atoms.
Zeolites are widely used as a catalyst in petro-chemical industries for cracking of hydrocarbons and isomerization, e.g., ZSM-5 used to convert alcohols directly in to Gasoline. Hydrated zeolites are used as ion exchangers in the softening of hard water.
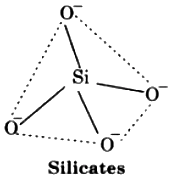
(ii) Silicates:- The basic structural unit of silicates is SiO4 in which silicon atom is bonded to four oxygen atoms in tetrahedron fashion. In silicates, either the discrete unit is present or a number of such units are joined via corners by sharing 1, 2, 3 or 4 oxygen atoms per silicate units. When silicate units are linked together, they form chain, ring, sheet or three dimensional structures. Negative charges on silicate structure is neutralised by positively charged metal ions. If all the four corners are shared with other tetrahedral units, it forms three-dimensional network. e.g., Feldspar, Zeolites, Mica and Asbestos. Man-made silicates are glass and cement.
(iii) Silicones:- Silicones are a group of organosilicon polymers, which have (R2SiO) as a repeating unit.
Silicones being surrounded by non-polar alkyl groups are water repelling in nature. They have high thermal stability, high dielectric strength and resistance to oxidation and chemicals. The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides, RnSiCl(4n) where R is alkyl or aryl group.

Silicones are used as greases, electrical insulators and for water proofing of fabrics. Being biocompatible, they are also used in surgical and cosmetic plants.