For a given mass of a gas, the amount of heat required to produce temperature difference is different for different substances. Let Q be the heat required to increase temperature by ∆T of gas of mass m, then the specific heat can be defined as follows :

The unit of specific heat in CGS system is cal g-1 C-1 and in MKS unit, it is represented as J kg-1 K-1 For example the specific heat for water is 4.2 × 103 J kg-1 K-1. The specific heat for gas can be from zero to infinity because if we compress a gas without heating it, then it increases the temperature.
For an adiabatic process,
Q = 0 and ∆T = positive, then C = 0
If on heating the gas, the gas expands such that there is no increase in the temperature, i.e., for isothermal process, then, from equation
Generally, to determine the amount of specific heat of a gas, pressure or volume is kept constant. Generally, there are two types of specific heats :
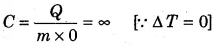
- Specific Heat at Constant Volume
- Specific Heat at Constant Pressure