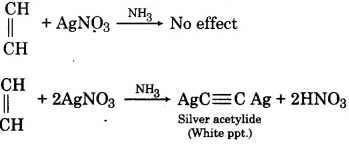
(1) When ammonical silver nitrate solution is added to ethylene, there is no effect. While when ammonical silver nitrate solution is added to acetylene it forms white precipitate of silver acetylide.
(2) Ethane and ethyne can be distinguished by bromine test. When bromine water is added to ethane, there is no effect. Whereas, when bromine water is added to ethyne, it gets decolourized.

(3) When a 5% solution of bromine in carbon tetrachloride is added to an organic compound, it gets decolourized. This indicates the presence of unsaturation in compound. While saturated hydrocarbon do not give this test.

(4) When ammonical silver nitrate is added to an organic compound, 1-butyne will give a white precipitate. While 1-butene does not react.
