(3) (b) and (c) are true
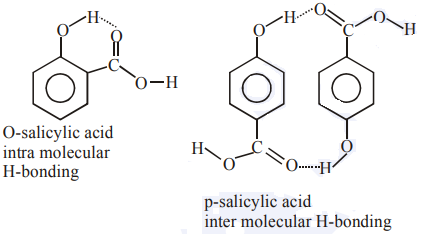
(a) B will be more crystalline due to more inter molecular interactions hence more efficient packing.
(b) B will have higher boiling point due to higher intermolecular interactions.
(c) B will be more soluble in water than A as B will have more extent of H-bonding in water
So all three statements are correct
{Solubility date ⇒ O-salicylic acid = 2g/L
P-salicylic acid = 5g/L}