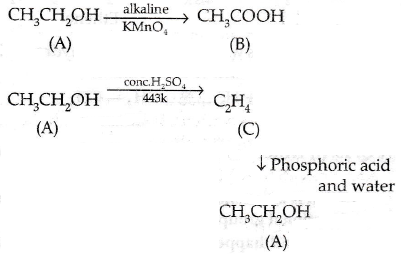
Since C gives an addition reaction with Br2 and H2 and so it is an unsaturated hydrocarbon. It gives an alcohol with a phosphoric acid and water i.e., CH3CH2OH. So C is ethene. Thus, A is ethanol which on oxidation with alkaline KMnO4 gives ethanoic acid. So we can conclude that A is ethanol, B is ethanoic acid and C is ethene.