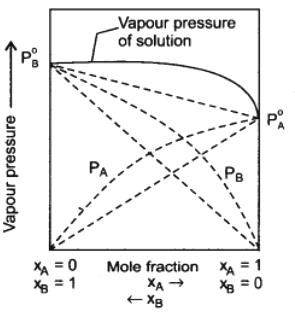
Some non-ideal solutions show positive deviation from Raoult’s law. Consider a solution of two components A and B. If A-B interactions in the solution are weaker than the A – A and B – B interactions in the two liquids forming the solution, then the escaping tendency of molecules A and B from the solution become more than in pure liquids.
The total vapour pressure will be greater than the corresponding vapour pressure as expected on the basis of Raoult’s law. This type of behaviour of solution is called positive deviation from Raoult’s law. The boiling point of such solutions are lowered.
Mathematically,
PA < PA0 xA
PB < PB0 xB
The total vapour pressure is less than PA + PB

Examples of solutions showing positive deviations
1. Ethyl alcohol and water
2. Benzene and acetone
3. Ethyl alcohol and cyclohcxanc
4. Carbon tetrachloride and chloroform.