B, has ten electrons but it is paramagnetic in nature.
It can be explained by its molecular orbital diagram:
5B = 2, 3 = 1s2 , 2s22p1
5B = 2, 3 = 1s2 , 2s2sp1
Molecular Orbital Configuration
(σ1s)2(σ*1s)2(σ2s)2 (σ*2s)2[(π2px)1(π2py)1]
(σ2pz)0[(π*2p)0 = (π*2p)0] (σ*2pz)0
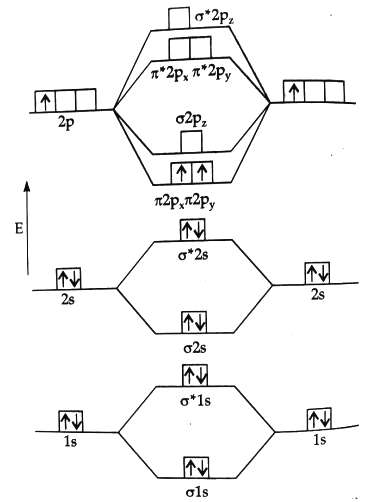
Since it contains two unpaired electron in π-binding molecular orbitals having equal energy. So, it is paramagnetic in nature.