1. Hydrogen gas is diatomic. According to MO theory. which is based on quantum mechanics H2 molecule can be represented in terms of the following diagram called M.O. diagram
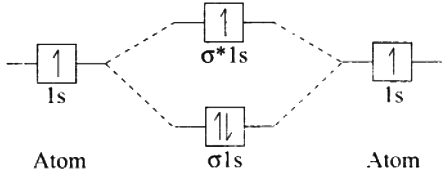
H – H. i.e.. H molecule has two atoms which are connected by 1 σ bond. So it is diatomic.
2. But in the case of inert gases. the valence shell is fully filled i.e.. an octet (8 electrons) (or) duplet (2 electrons) in case of Helium, due to which they are in monoatomic state and remain stable. So they do not combine with any atom (neither of same or of different elements). Due to this they do no exist in diatomic state and always exist in mono – atomic state.